Abstract
Background: Patient with hematological malignancies (HM) often experience worsening of quality of life (QoL) due to both the disease and its treatments. Impaired QoL may have negative impact on treatment outcomes leading to change in treatment decision-making. However, this has not been captured in a systematic manner in routine clinical practice and there is no specific instrument for such purpose. Thus, there is the need of a new patient-reported outcome (PRO) measure. The aims of this study were to identify issues important to patients with HM and to develop a new PRO measure for use in daily clinical practice.
Methods: Following FDA guidelines for the development of PRO measures, a conceptual framework was developed using preliminary literature search and discussions with clinicians and HM patients. Patients were interviewed to produce a comprehensive item pool reported as important by them. The generated items were discussed in a data definition panel meeting to select items to be included in the prototype version of the new instrument, HM-PRO. To examine the relevance, clarity and comprehensiveness of the items bank and appropriateness of the recall period, a 3-phase qualitative study was conducted: 1) 34 patients were asked to complete the HM-PRO of whom 8 were randomly selected for cognitive interviewing; 2) content validity panel meetings were conducted where a panel of experts (12 members) and a panel of patients (10) were asked to rate each item of HM-PRO for language clarity, completeness, relevance and scaling, followed by 2 panel discussion to reach consensus. Subsequently, a panel of experts was also asked to classify each item into its relevant disease type based on their clinical experience; and 3) A second draft of the instrument was developed and tested for relevance (cognitive interviews - n=26) using verbal probing and double interviewing technique.
Results: 129 patients [male=76; mean age = 61 years; SD=15.3; median age =65 years; age range =18-88 years; diagnosis - acute myeloid leukemia (AML n=18), acute lymphoblastic leukemia (ALL n=7), chronic myeloid leukemia (CML n=12), chronic lymphocytic leukemia (CLL n=11), multiple myeloma (MM n=21), aggressive non Hodgkin lymphoma (ANHL n=17), indolent NHL (INHL n=14), HL (n=11), myeloproliferative neoplasm (MPN n=10), and myelodysplastic syndrome (MDS n=8)] with mean duration of HM of 3.6 years (SD=4.3; and range=19 days-23 years) from 5 hematology centers were interviewed to identify the issues important to HM patients. The conceptual framework comprised of two main themes: QoL (impact); and symptoms. A prototype version of HM-PRO was developed after data definition panel meeting with 34 items in impact category (Part A) and 23 disease symptom items (Part B). A 12-member panel of experts and 10-member panel of patients, rated the HM-PRO items (both Parts A & B) for their language clarity, completeness, relevance and scaling and subsequently discussed them in 2 separate panel meetings to reach consensus. Content validity Index (CVI) showed strong agreement (0.84-0.98) between the ratings of the panel members for the four criteria.
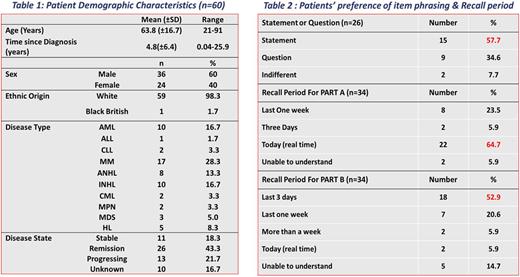
Sixty out-patients (Table 1) were recruited for the pilot testing of whom 34 were involved in cognitive interviews. 15 (58% - n=26) patients preferred the HM-PRO items to be phrased as 'statements', the majority (65% - n=34) preferred 'today' (at the moment) as the recall period for part A (i.e. impact) and 53% (n=34) the 'last 3 days' for Part B (i.e. symptoms) (Tables 2). Nearly all patients found the HM-PRO items relevant and felt that all issues important to them were covered. There were no preference of paper color of the instrument, but when asked to choose between two colors, green was preferred by 73%.
Conclusions: The findings of this study render the HM-PRO as a novel PRO instrument in HM for use in clinical practice. It possesses a strong content validity in different HMs, includes all the issues important to patients and its statements are easy to read, understand and respond to spontaneously. The recall period of 'today' for Part A and 'last 3 days' for Part B of the HM-PRO are the preferred recall periods from the patients' perspective. Patients expressed that responding to HM-PRO items as statements is more personalised. The HM-PRO items represent QoL and symptoms relevant/applicable to all 10 HMs. HM-PRO will undergo further psychometric testing to support its psychometric properties across different types of HMs.
Salek: EHA: Other: Educational and Travel Grant. Oliva: Amgen Inc.: Consultancy; Janssen: Consultancy; Novartis: Consultancy; Celgene: Consultancy; La Jolla: Consultancy. Fielding: Pfizer: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Baxalta: Membership on an entity's Board of Directors or advisory committees. Collins: Pfizer: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Speakers Bureau; Amgen: Research Funding; ADC Therapeutics: Research Funding; MSD: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Speakers Bureau; Celleron: Consultancy; Bristol-Myers Squibb: Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal